
Background:
Enzymes, as one of the most effective biocatalysts in nature, possess excellent regio- and chemo-selectivity, allowing precise control of biological processes under mild conditions. The diversity of identified enzyme classes enables the execution of almost all transformation reactions required to sustain life processes. Despite exhibiting superior catalytic functionality in cellular environments, many enzymes can only maintain high stability and activity within the crowded intracellular milieu. Hence, it is crucial to research and develop strategies for stabilizing enzyme molecules.
Immobilization techniques for enzymes are typically based on strategies that facilitate recovery and reduce adverse external influences to enhance operational stability. However, this approach often leads to reduced catalytic efficiency due to effects of surface interactions. Recent studies have indicated that by precise design and control, certain immobilized enzyme carriers can enhance enzymatic activity while maintaining stability. Among them, Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs) are two promising materials due to their tunable chemical properties and pore structures. Although existing research has shown that customized MOF and COF encapsulation can enhance the apparent activity of enzymes, the mechanisms by which carriers affect enzyme conformation remain insufficiently understood, especially the impact of shape and size complementarity between rigid scaffolds and flexible enzyme catalysts is currently a blank space.
Recently, the research team of Academician Tan Tianwei from Beijing University of Chemical Technology and Professor Lv Yongqin reported an innovative synthesis strategy based on "physical imprinting." They used enzyme molecules as templates to construct "imprint cavities" in Metal-Organic Framework materials (MOFs) that match the size and shape of enzyme molecules, significantly enhancing the enzyme's apparent activity and operational stability. The study also elucidated the mechanism of improving enzyme activity and stability through molecular recognition microenvironments.
Research Content:
The method of constructing tunnels in Metal-Organic Framework materials that match the size and shape of enzymes based on "physical imprinting" is shown in the diagram below. Enzyme "templates" are introduced during the MOF synthesis process to arrange the framework, where enzyme molecules act as scaffolds, mediating the coordination assembly of metal nodes and organic ligands. After calcination at 350°C to remove the enzyme template, customized "imprint cavities" matching the size and shape of enzymes are tailored in the MOF. These nano-cavities exhibit specificity and selectivity towards template enzyme molecules based on shape and size complementary structures. The catalytic activity of the novel carrier-bound enzyme is 16.7 times that of free enzymes, while the catalytic efficiency (kcat/KM) is 14.1 times higher than free enzymes, both reaching the highest reported values in the literature. The residual enzyme activity of the immobilized enzyme remains above 65% under high temperature at 95°C, pH=2, pH=10, organic solvents such as methanol and acetone, as well as trypsin treatment. Even after 18 consecutive uses, the residual enzyme activity remains above 80%. Various techniques including solid-state UV-vis, Electron Paramagnetic Resonance (EPR), Fourier Transform Infrared Spectroscopy, and solid-state Nuclear Magnetic Resonance elucidated the mechanism of enhancing enzyme activity and stability through molecular recognition microenvironments. The "imprint cavities" constructed by physical imprinting reshape the enzyme's active sites in the customized confined space through restricted folding dynamics and cofactor coordination, finely controlling the enzyme's microenvironment and stabilizing the active conformation of the enzyme.
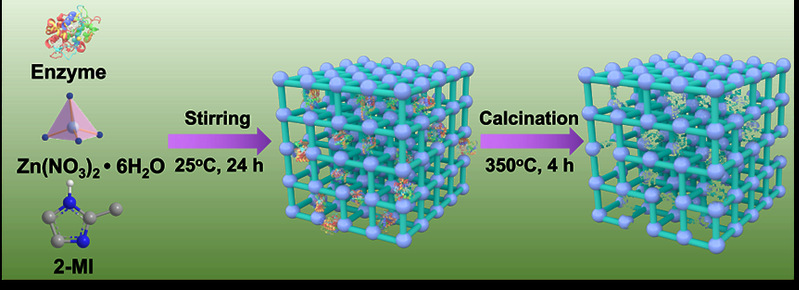
Fig1. Constructing "imprinted cavities" based on "physical imprints" in Metal-Organic Frameworks (MOFs) to match the size and shape of enzyme molecules.
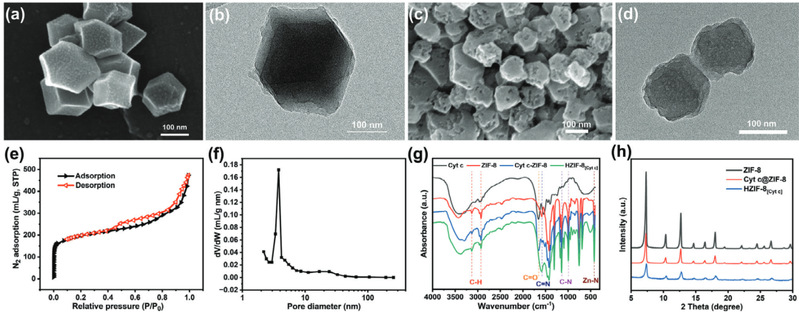
Fig2. (a) SEM and (b) TEM images of Cyt c@ZIF-8 composite, SEM and TEM images of HZIF-8[Cyt c], N2 adsorption isotherms, and pore size distribution graphs (c-f), Infrared spectra of Cyt c, ZIF-8, Cyt c@ZIF-8 composite, and HZIF-8[Cyt c] (g), Powder XRD diffraction patterns of ZIF-8, Cyt c@ZIF-8 composite, and HZIF-8[Cyt c] (h)
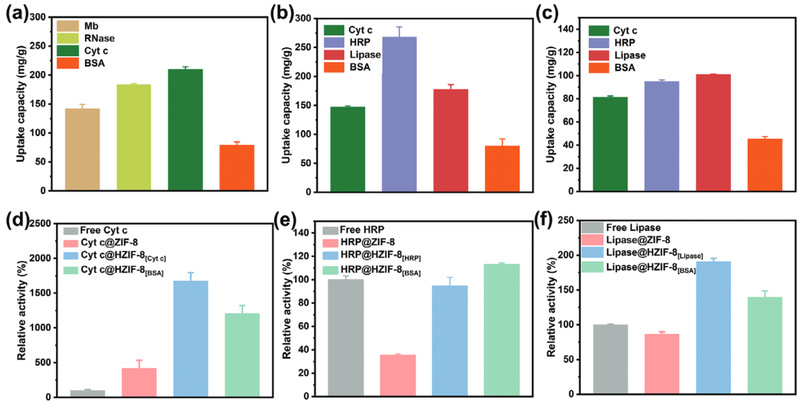
Fig3. Adsorption capacities of HZIF-8[Cyt c], HZIF-8[HRP], and HZIF-8[Lipase] towards various enzyme molecules (a-c), as well as relative enzyme activities after enzyme immobilization (d-f).
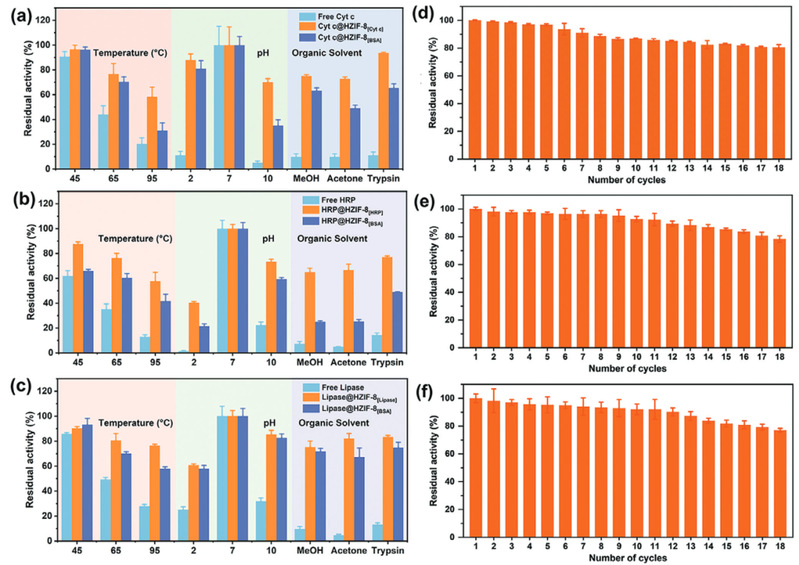
Fig4. Stability and reusability of free and immobilized enzymes. (a, d) Cytochrome c; (b, e) Horseradish peroxidase; (c, f) Lipase
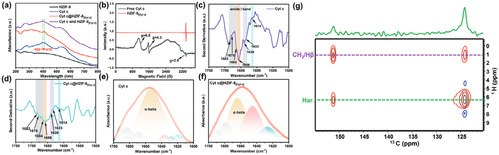
Fig5. Mechanism of enhancing enzyme activity and stability by analyzing molecular identification microenvironments based on solid-state UV-vis, electron paramagnetic resonance (EPR), Fourier transform infrared spectroscopy, and solid-state nuclear magnetic resonance spectroscopy.
Author Introduction:

Dr. Lv Yongqin is a professor and doctoral supervisor at Beijing University of Chemical Technology. He is a recipient of the National Outstanding Youth Fund. He serves as the Deputy Director of the Beijing Key Laboratory of Bioprocess and the Deputy Director of the International Cooperation Joint Laboratory of Biological Energy under the Ministry of Education. Lv Yongqin graduated from the School of Materials Science and Engineering of Beijing University of Chemical Technology in 2005 with a Bachelor's degree. In December 2010, he obtained his Ph.D. in Chemical Engineering and Technology from Beijing University of Chemical Technology under the guidance of Academician Tan Tianwei. From November 2008 to February 2010, he was sponsored by the China Scholarship Council to study and exchange as a joint doctoral student in the Materials Science Department at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in Australia. From January 2011 to December 2012, he worked as a postdoctoral researcher at the Department of Chemistry at the University of California, Berkeley and Lawrence National Laboratory in the United States. In January 2013, he was employed by Beijing University of Chemical Technology's School of Life Science and Technology under the "Talent Introduction" program. He has received awards such as the Hou Debang Award for Chemical Science and Technology, the second prize of the Natural Science Award of the Ministry of Education, and the first prize of the Technical Invention Award of Petrochemical Industry Association, among others. His main research interests include regulating confined microenvironments to promote biocatalysis, photo/electro-driven biocatalytic carbon fixation, and bio-inspired antibody engineering. He has published over 60 SCI papers as first or corresponding author in journals such as PNAS, J. Am. Chem. Soc., Matter, Nano Lett., Adv. Sci., Prog. Energ. Combust., Biotechnol. Adv., applied for more than 20 patents, and led more than 10 research projects, including 9 at the national level. He is a member of the Biotechnology Committee of the China Society of Biotechnology, as well as a member of the Youth Working Committee of the China Association of Chemical Engineering. Lv Yongqin also serves as a guest editor for Biotechnology Advances, a young editor and guest editor for Frontiers of Chemical Science & Engineering, an editor for Microchimica Acta, a young editor for Bioresources and Bioprocessing, an editor and guest editor for Synthetic and Systems Biotechnology, an editor for Biotechnology Notes, and an editor of "Bioprocess Engineering".


