The CRISPR-Cas system is widely found in bacteria and archaea, and is an adaptive immune system in prokaryotes that is used to defend against invasion by exogenous nucleic acids such as viruses and plasmids. However, in 2013, a researcher discovered the type I-F CRISPR-Cas system in the ICP1 phage. It remains to be investigated how the CRISPR-Cas system of phage compares to the bacterial CRISPR-Cas system.
On 8 July 2024, Feng Yue's group at our university, in collaboration with Yang Maojun's group at Tsinghua University, published a paper entitled "Cas1 mediates the interference stage in a phage-encoded CRISPR-Cas system" in Nature Chemical Biology. Cas1 mediates the interference stage in a phage-encoded CRISPR-Cas system" and "An alternative mechanism for recruiting Cas2/3 in a phage-encoded CRISPR-Cas system" in Nature Chemical Biology. The research brief "An alternative mechanism for recruiting Cas2/3 in a phage-encoded CRISPR-Cas system" and "An alternative mechanism for recruiting Cas2/3 in a phage-encoded CRISPR-Cas system" report a unique mechanism for recruiting Cas2/3 in the ICP1 phage-encoded CRISPR-Cas system to degrade target DNA.

In this paper, they elucidated the molecular mechanism of the novel recruitment of Cas2/3 by the ICP1 CRISPR-Cas system complex through various means, including structural biology, biochemistry, and phage science. Firstly, they found that ICP1 Cas1 can bind ICP1 Csy or Csy-dsDNA complexes, while Cas1 of Pseudomonas aeruginosa does not bind its Csy or Csy-dsDNA complexes; secondly, after binding ICP1 Csy-dsDNA, the ICP1 Cas1-Cas2/3 complexes can form stable Csy-dsDNA- Cas1-Cas2/3 complex, which was resolved by the collaboration of Feng Yue's group and Yang Maojun's group (Figure 1). In this complex, the Cas1-Cas2/3 complex is located in the centre, and each side binds a Csy-dsDNA complex, with a total molecular weight of about 1 MDa, which is also the first complex structure in which the I-F type CRISPR-Cas system binds Cas2/3, while Cas1 will gradually be dissociated from Cas2/3 after the Pseudomonas aeruginosa Cas1-Cas2/3 binds its Csy-dsDNA. dissociate from Cas2/3, and eventually only Csy-dsDNA-Cas2/3 complexes can be formed; finally, in vivo and in vitro activity experiments proved that ICP1 Cas2/3 can better degrade the DNA targeted by Csy complexes only in the presence of Cas1, while Pseudomonas aeruginosa Cas1 hardly affects the degradation of the target DNA by Cas2/3.Combining the above results Feng Yue's group proposed a new mechanism of ICP1 CRISPR-Cas system by which Cas1 recruits Cas2/3 for target DNA degradation.
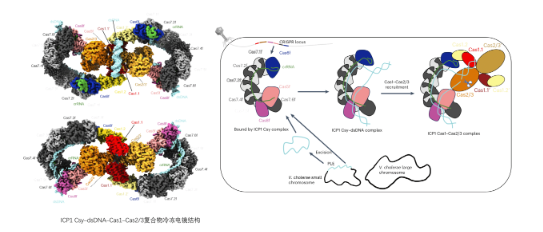
Figure 1 Interference mechanism of ICP1 I-F type CRISPR-Cas system
In summary, Cas1 mediates target DNA degradation in the ICP1 CRISPR-Cas system by linking the Csy complex and Cas2/3. This finding demonstrates the critical role of Cas1 in the interference phase of the CRISPR-Cas system and breaks the long-standing conventional wisdom in the field that it only plays a role in the adaptation phase. Dr Lai-Hsing Zhang of Tsinghua University, Dr Hao Wang of Beijing University of Chemical Technology, Dr Jian-Wei Zeng of Tsinghua University, Dr Xue-Li Cao of Beijing University of Chemical Technology and graduated master's student Zheng-Yu Gao are the co-first authors of this paper, while Professor Yue Feng of Beijing University of Chemical Technology, Associate Professor Yi Zhang and Professor Mao-Jun Yang of Tsinghua University are the co-corresponding authors of this paper.
Link to original article:
https://www.nature.com/articles/s41589-024-01659-5
https://www.nature.com/articles/s41589-024-01667-5
Yue Feng is a professor and doctoral supervisor at Beijing University of Chemical Technology, and a recipient of the National Excellent Youth Fund (2018). He mainly uses biochemistry and molecular biology, structural biology, cell biology and other means to carry out research on the mechanism of microbial-host immune system interactions. He has published a total of 50 SCI papers, including 27 papers with corresponding authors (including co-authors) in Nature, Cell, Mol Cell, Nat Chem Biol, PNAS, Nat Plants, Nat Commun, Nucleic Acids Res and other internationally renowned journals. He has been in charge of many national and provincial projects. He has been awarded the honours of National Young Position Master (2020), Beijing Outstanding Young Talent (2020), Second Prize of Beijing Science and Technology Progress Award (2018), China's Top 10 Emerging Science and Technology Figures (2018), and Beijing Science and Technology Rising Star (2019).


