Abstract:
As a frontline “life-saving drug” in emergency medicine, epinephrine faces rigid and massive market demand. However, traditional chemical synthesis routes have long been hampered by persistent challenges including inefficient chiral resolution, use of highly toxic reagents, and poor atom economy. Recently, Zhang Yifei's team at Beijing University of Chemical Technology published groundbreaking research in Green Chemistry, designing an entirely novel in vitro multi-enzyme cascade pathway. Through ingenious “tunnel engineering” modifications of enzyme molecules and modular continuous-flow reactor design, they successfully achieved green manufacturing of epinephrine with high optical purity (STY reaching 2.17 g/L/h). This article provides an in-depth analysis of this technological innovation, from the logic of enzyme modification to the process integration strategy.
Core Content:
01 Background: The Leap from “High Pollution” to “Green Smart Manufacturing” Industrial EPI production has long faced a dilemma: extraction from animal and plant sources yields extremely low efficiency (only kilogram-scale products from tons of raw materials), while chemical total synthesis, though scalable, typically produces racemic mixtures. Subsequent chiral resolution is not only time-consuming but also requires toxic reagents like chloroacetyl chloride. How can biocatalysis' high stereoselectivity and environmental friendliness replace traditional methods? The core challenge lies in constructing a thermodynamically viable enzymatic pathway that also addresses the high cost of cofactors.
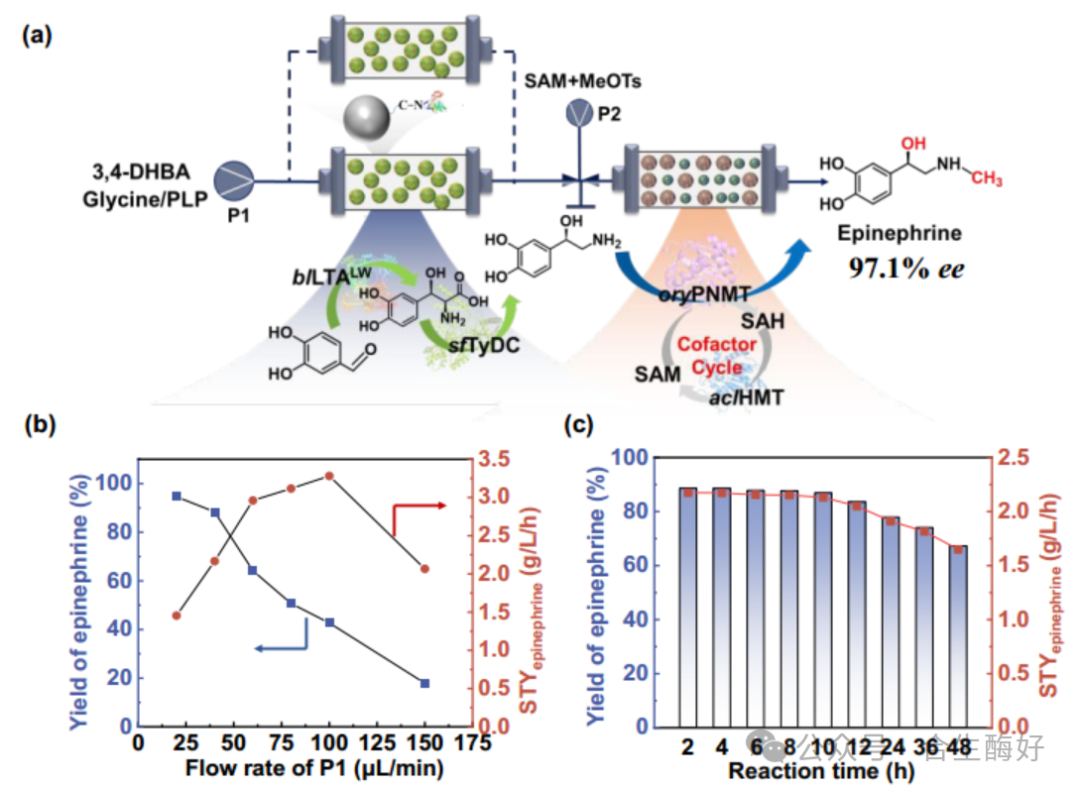
02 Core Innovation 1: Retro-biosynthetic Analysis and Pathway Reconstruction. Using RetroBioCat tools and literature review, the research team evaluated three potential routes, ultimately identifying a four-enzyme cascade pathway (Route 1): L-Threonine Aldolase (LTA): Catalyzes the condensation of 3,4-dihydroxybenzaldehyde (3,4-DHBA) with glycine to form the intermediate L-threo-DOPS (tuxidopa). Tyrosine decarboxylase (TyDC): Decarboxylates DOPS to form norepinephrine. Phenethylamine N-methyltransferase (PNMT) & Halide methyltransferase (HMT): Complete the final methylation step and introduce a SAM recycling system. This pathway circumvents the oxygen dependency inherent in traditional biosynthesis (hydroxylases typically require oxygen and exhibit low water solubility), making it theoretically more suitable for industrial scale-up.
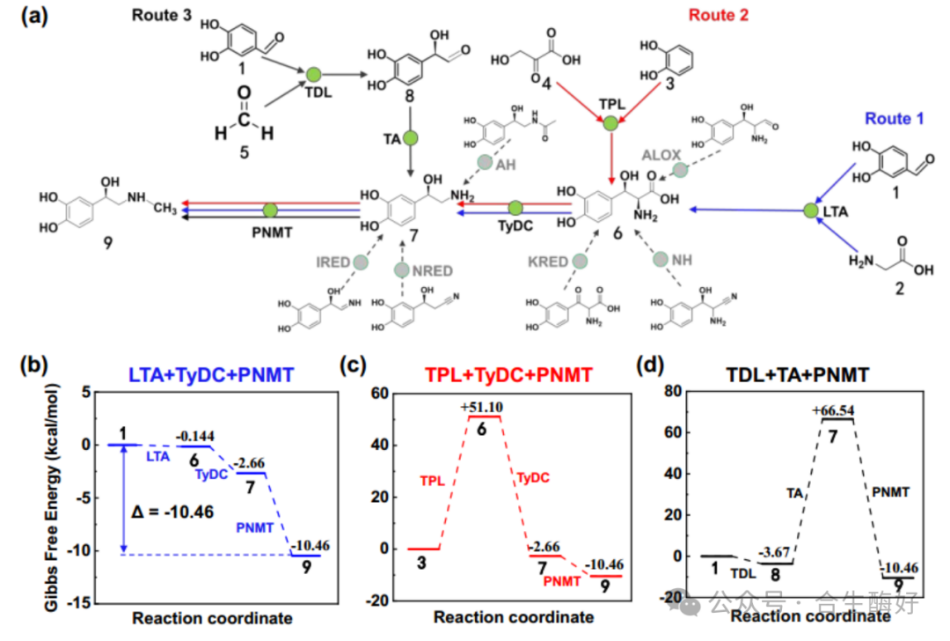
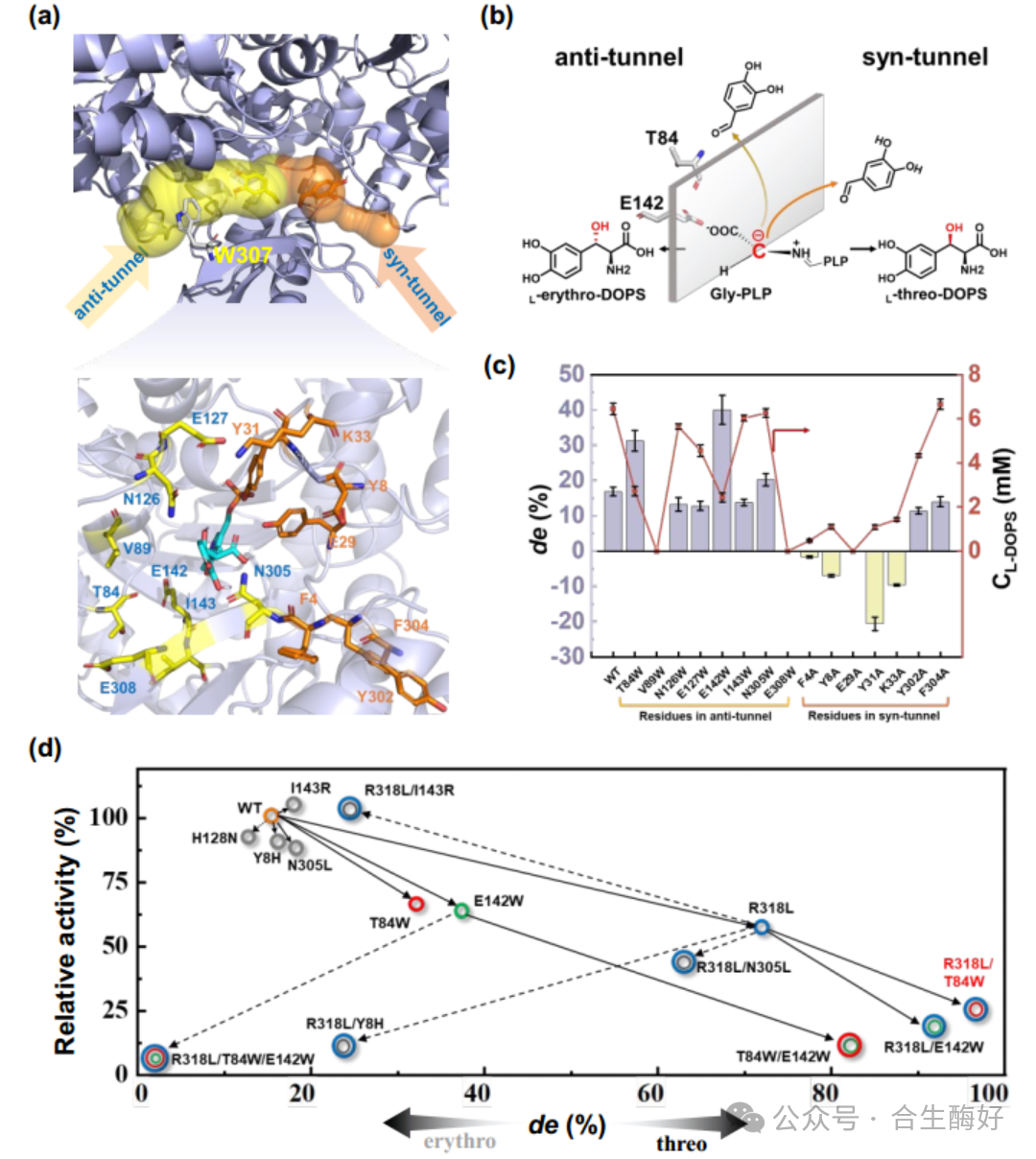
03 Core Innovation II: “Tunnel Engineering” Modification of Enzyme Molecules. This represents the most remarkable technical achievement of this study. The rate-limiting step lies in the initial LTA catalysis. Wild-type LTA exhibits poor stereoselectivity, simultaneously producing both L-threo and L-erythro configurations. To achieve a single configuration, the research team eschewed conventional random mutation and instead adopted a structure-guided tunnel engineering strategy. Problem Diagnosis: CAVER 3.0 analysis revealed two substrate entry channels (syn-tunnel and anti-tunnel) in the LTA active site. The channel through which the substrate enters directly determines the stereochemical configuration of the product. Modification Strategy: Blocking the Anti-tunnel: Introducing large hydrophobic residues (e.g., W307) increases steric hindrance, blocking the pathway for byproduct formation. Syn-tunnel Reshaping: Introduced the key R318L mutation. This eliminates the original salt-bridge interaction, reorients glycine's Cα toward the syn-tunnel, and locks substrate conformation via a novel hydrogen-bond network with H128, Y8, and Y31. Final Outcome: By stacking mutations, the triple mutant blLTA-LW (T84W/E142W/R318L) was constructed. This mutant maintained exceptionally high stereoselectivity across a broad pH range, with de values soaring from 18.5% in wild-type to 97.3%, while retaining 25% catalytic activity.
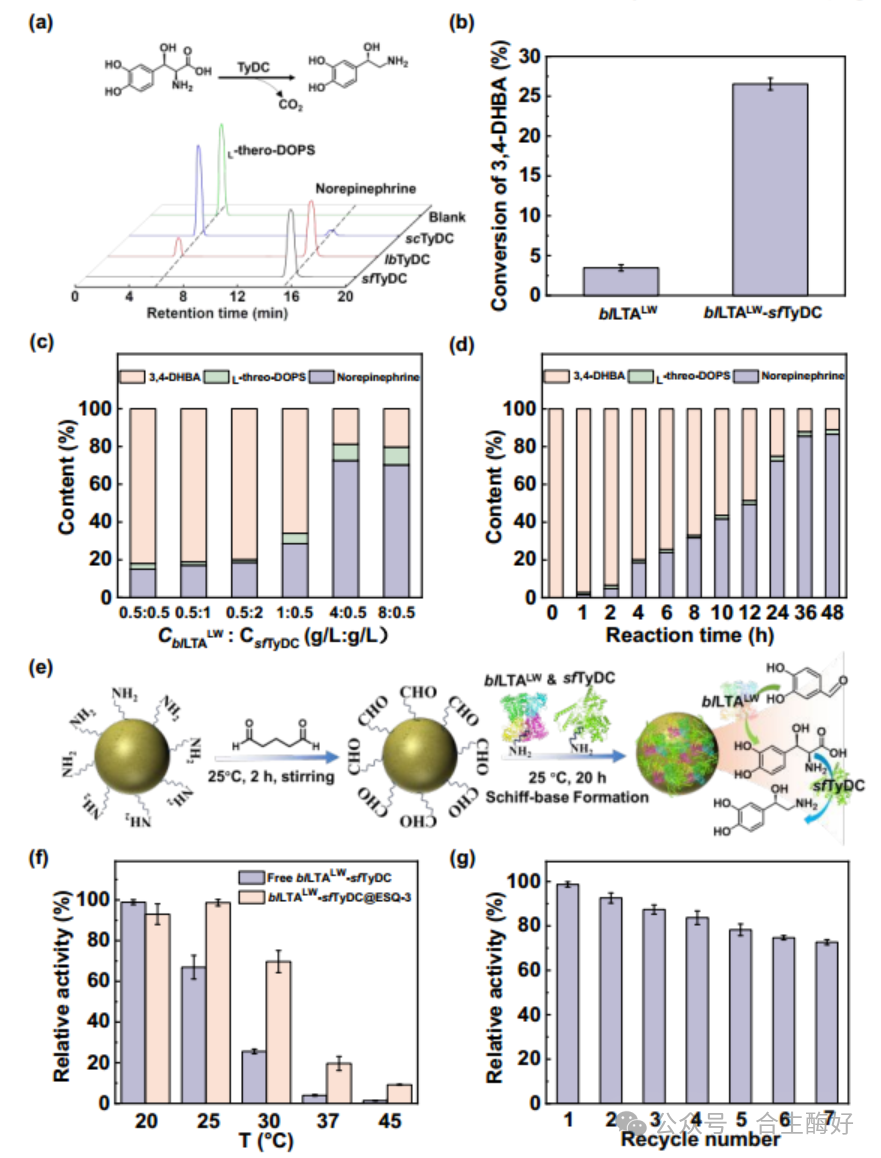
04Core Innovation 3: The process integration of the dual-module continuous flow system addressed enzyme performance issues. The next step was tackling reaction equilibrium and cofactor costs. The research team devised a “dual-module” strategy:
Module 1: Norepinephrine Synthesis (Driving Equilibrium) Since the aldol reaction catalyzed by LTA is reversible, conversion rates are typically limited. The team ingeniously introduced highly active sfTyDC (from Streptococcus faecium), which rapidly decarboxylates the intermediate from DOPS to norepinephrine. This decarboxylation releases CO₂, acting as a “thermodynamic precipitant” that forces the upstream reversible reaction forward, boosting conversion from 3.4% to 87.9%.
Module II: Methylation and In Situ SAM Regeneration (Cost Reduction) SAM (S-adenosylmethionine) is prohibitively expensive for direct stoichiometric use. The team constructed a PNMT + aclHMT dual-enzyme coupling system. Replacing highly toxic methyl iodide with the inexpensive and less toxic methyl donor MeOTs (methyl p-toluenesulfonate) enabled in situ SAM regeneration cycles. Key technical points: Discovery of substrate inhibition (high norepinephrine concentrations inhibit PNMT) provided the rationale for introducing a continuous-flow reactor—lifting inhibition by controlling substrate concentration and residence time.
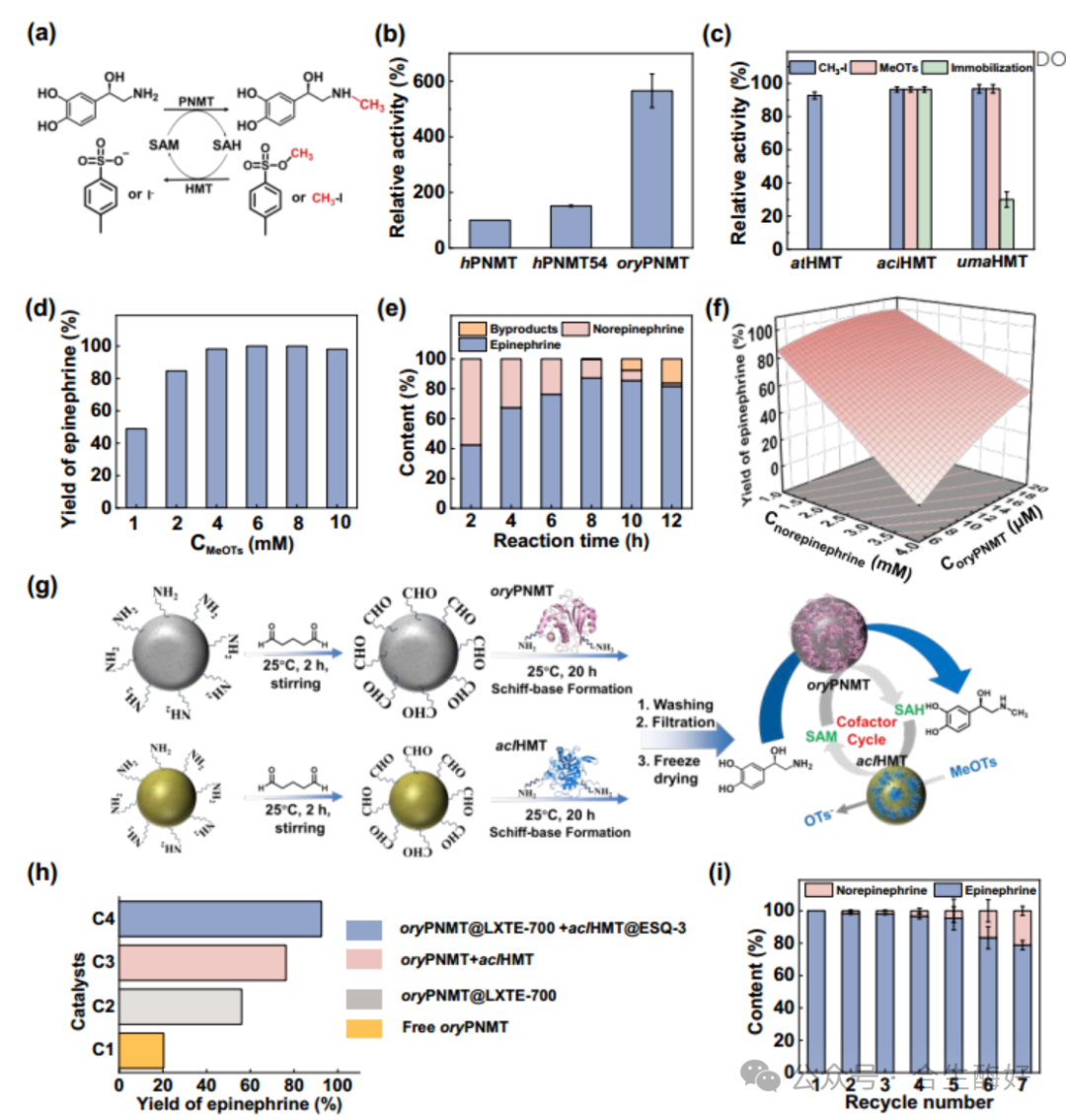
05 Results Data: Industrialization Potential Validation Ultimately, the team immobilized enzymes on resins (ESQ-3 and LXTE-700) and assembled them into packed-bed reactors. Spatio-temporal yield (STY): 2.17 g L⁻¹ h⁻¹. Optical purity: Product ee value as high as 97.1%. Stability: Conversion rate remained above 67% after 48 hours of continuous operation.
Industrial Prospects and Investment Value
As observers in the synthetic biology field, we believe this work represents not only an outstanding Green Chemistry paper but also exemplifies the practical implementation of the “Biocatalysis + Flow Chemistry” technological trend.
Precision in Enzyme Engineering: The shift from random evolution to rational design, particularly the fine-tuned regulation of the enzyme's internal “substrate channel,” demonstrates the immense power of integrating computational biology with enzyme engineering. This represents a key strategy for future development of highly stereoselective enzymes.
Victory in Atomic Economy: Through modular design integrating multi-step reactions, the approach not only enables low-cost recycling of the cofactor (SAM) but also eliminates the need for intermediate separation and purification, substantially enhancing atomic economy.
Bridge from Lab to Plant: The incorporation of continuous flow technology resolves common product/substrate inhibition issues in enzyme catalysis while significantly improving mass transfer efficiency. For APIs like epinephrine, which demand high value-added and high-purity standards, this manufacturing model holds immense commercial potential.
Conclusion and Outlook:
Although the current STY still has room for improvement to meet large-scale industrialization requirements (typically >10 g/L/h), and LTA's catalytic activity requires further optimization, this pathway has demonstrated the core competitiveness of biomanufacturing in synthesizing complex chiral drugs. Looking ahead, with iterative improvements in enzyme activity and optimized fluidic processes, we have every reason to believe that greener, more efficient EPI “bioreactors” are on the horizon.


