
01 Abstract
This study summarizes how, based on the highly catalytic GALS M3 variant (T87A/A416T/W463I), computer-aided design strategies were employed to further improve the thermal stability and activity of glyoxylate synthase (GALS). After two rounds of protein engineering, the optimal variant GALS M5 (GALS M3-A381P/K290P) was obtained, exhibiting a melting temperature (Tm) elevated to 63°C and an initial activity as high as 2204.55 U/g—the highest reported activity and thermal stability for GALS to date. Additionally, the mutant GALS M3-S61A exhibited thermoactivation, with activity increasing 1.4-fold after 3-hour incubation at 50°C. Molecular dynamics simulations revealed the mechanism by which the mutation enhances structural rigidity. This study provides new insights for synergistic optimization in enzyme engineering.
02 Research Background
Glyoxylate synthase (GALS) is a key biocatalyst that catalyzes the condensation of two formaldehyde molecules (FALD) to form glyoxylate (GALD). holding broad application prospects in converting C1 resources (e.g., methanol, CO₂) into bulk chemicals (e.g., vinyl alcohol, PHB, L-sorbitol) and the food additive D-erythritol. However, industrial utilization of GALS is constrained by its insufficient thermal stability: the enzyme readily inactivates at elevated temperatures, and the toxicity of formaldehyde in reaction systems demands high enzyme activity for rapid substrate conversion. Previous studies primarily enhanced GALS activity through directed evolution or semi-rational design (e.g., the GALSF397Y/C398M variant reported by Zhang et al.), with limited improvements in thermal stability. Our research team previously obtained the GALS M3 variant via tunnel engineering, achieving a 15.97-fold increase in catalytic efficiency, yet its thermal stability remains suboptimal. Therefore, synergistically enhancing both thermal stability and activity of GALS emerges as a critical scientific challenge.
03 Research Questions This study aims to address the following questions:
1. Trade-off between thermal stability and activity: In traditional enzyme engineering, increased stability often comes at the expense of activity. Can this trade-off be overcome?
2. Mutant site selection: How can key residues (i.e., “hotspots”) affecting GALS stability be precisely identified?
3. Mechanistic Understanding: How do mutations influence enzyme structural dynamics and catalytic behavior at the molecular level? For example, what causes the phenomenon of thermal activation?
04 Research Approach
This study employs a computer-aided rational design strategy combined with experimental validation and molecular dynamics simulations, following these steps:
1.Hotspot Prediction: Analyze the crystal structure of GALS M3 (predicted via AlphaFold2) using the FireProt 2.1 online server. Screen potential mutation sites based on B-factor (≥40 Ų) and folding free energy change (ΔΔG ≤ -1 kcal/mol). Ultimately select 16 candidate sites, such as K290 and A381, located in highly flexible regions (e.g., loop regions).
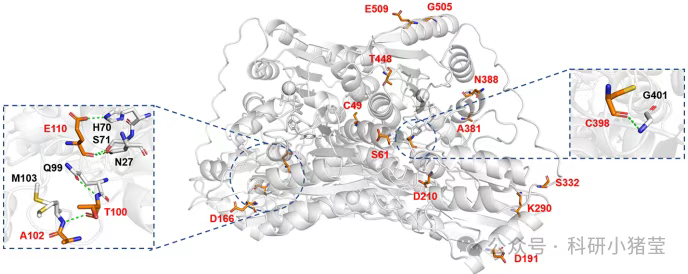
2. Single-Point Mutant Library Construction: Sixteen single-point mutants were constructed via site-directed mutagenesis in the pET-22b(+) vector. After expression and purification, their melting temperatures (Tm) and relative activities were measured. Results showed that some mutants (e.g., A381P and S61A) increased Tm while maintaining activity.
3. Combination Mutation: Using the A381P variant (GALS M4) with optimal activity and stability as a template, a second round of combination mutation yielded the double-point mutant GALS M5 (A381P/K290P). Attempts at triple-point mutation were made, but only GALS M5-S61A and GALS M5-E509F were successfully expressed.
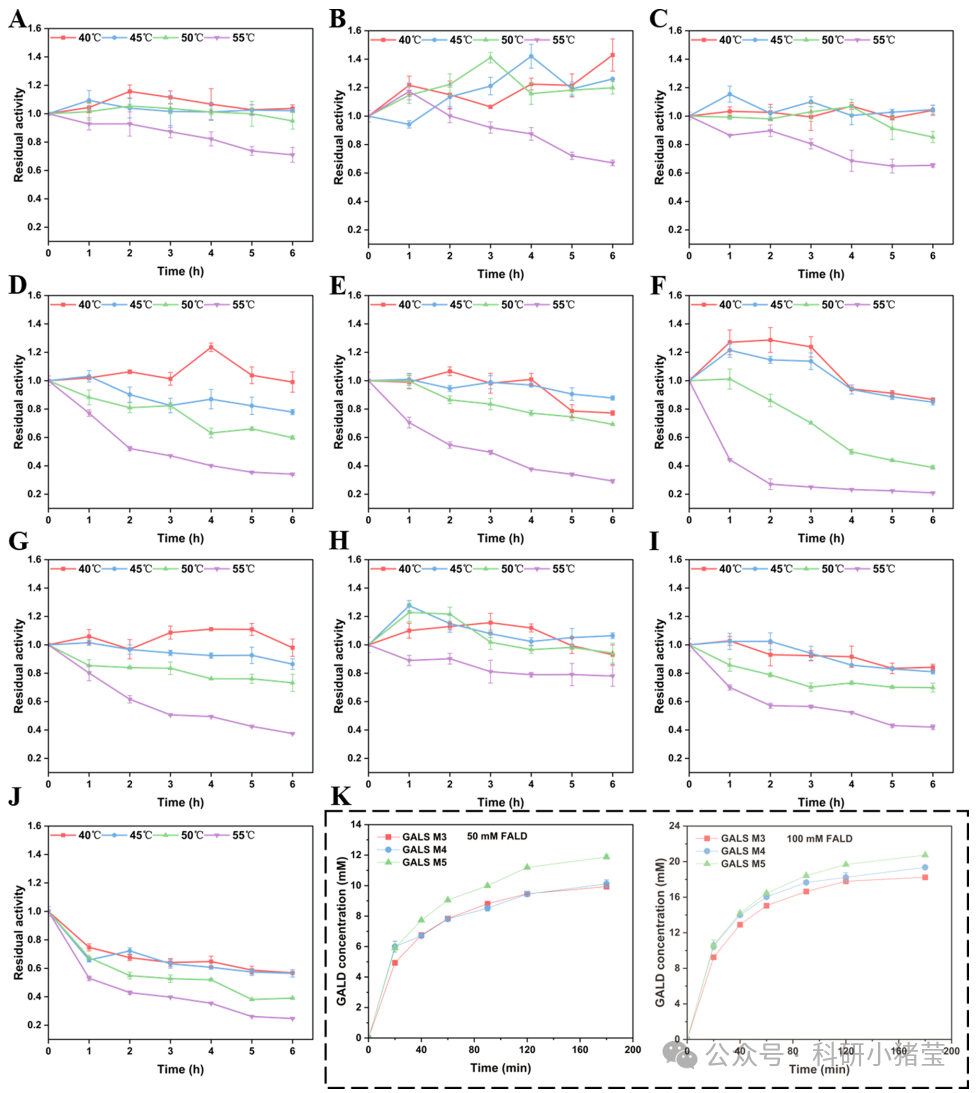
4. Enzymatic Characterization: Kinetic parameters (Km, kcat), thermal stability (residual activity over time), and catalytic performance (glyoxalate yield) were measured for variants. Experiments were conducted at 40°C–55°C to assess time-dependent activity changes.
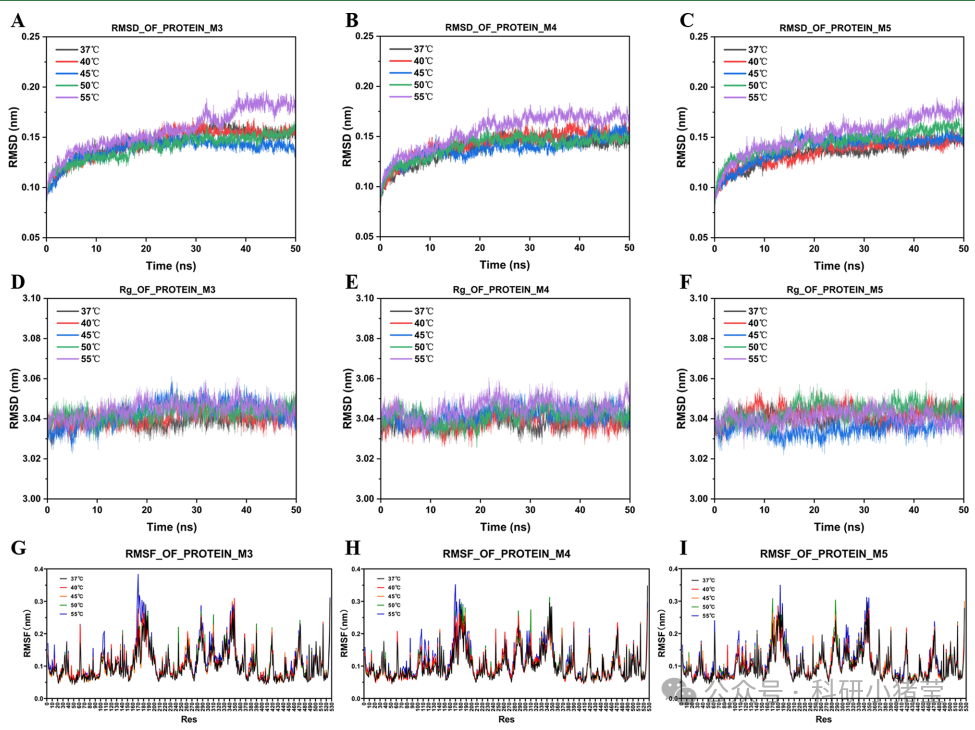
5. Molecular dynamics simulations: 50 ns simulations at 37°C–55°C using GROMACS software analyzed protein backbone RMSD, radius of gyration (Rg), residue fluctuation (RMSF), and free energy landscapes to elucidate the effects of mutations on structural rigidity and conformational dynamics.
05 Research Conclusions and Innovations
1. Synergistic Performance Enhancement: The GALS M5 variant achieves synergistic improvement in thermal stability (Tm=63°C) and activity (2204.55 U/g). It retains 100% activity after 6-hour incubation at 50°C and demonstrates excellent conversion efficiency at high substrate concentrations (100 mM formaldehyde).
2. Thermal Activation Phenomenon: GALS M3-S61A exhibits significant activity enhancement after incubation at 40°C–50°C. Mechanistic analysis indicates high-temperature-induced conformational rearrangement generates a high-affinity catalytic state, rather than protein unfolding.
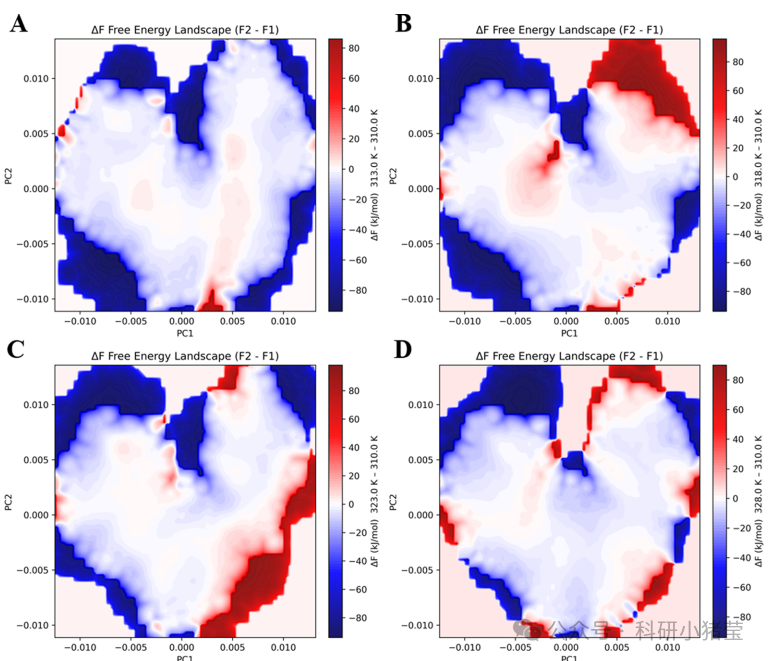
3. Molecular Mechanism: MD simulations reveal that mutations in GALS M5 (e.g., A381P and K290P) enhance loop rigidity and reduce thermal fluctuations (RMSD stabilized at 0.165 nm), whereas the thermoactivated variant increases catalytic efficiency through increased conformational diversity.
4. Innovation Highlights: Methodological Innovation: Integrating computational prediction (FireProt) with experimental screening enables rapid rational design. Application Prospects: GALS M5 provides an efficient module for C1 biorefining, potentially accelerating industrialization. Theoretical Contribution: First discovery of thermal activation behavior in GALS enzymes, offering new insights into enzyme kinetics research.


