Intracellular bacterial infections are associated with many serious infectious diseases such as tuberculosis, endocarditis, osteomyelitis, necrotizing pneumonia, and sepsis. Clinical treatment is difficult to eradicate intracellular bacteria due to various reasons: (i) poor penetration of antibiotics through cell membranes leading to low intracellular accumulation and short retention time; (ii) harsh acidic and hydrolytic environment within lysosomes reducing antibiotic antimicrobial activity; (iii) dormant intracellular bacteria exhibiting tolerance to lethal antibiotic concentrations; (iv) bacterial escape from lysosomes and hiding within the cytoplasm to evade antibiotic clearance. To combat these resilient bacteria, delivering antibiotics precisely to the bacterial residing site through targeted intracellular bacteria is a feasible approach, albeit scantily reported.
To this end, Professor Wang Xing's research group at the School of Life Science and Technology, Beijing University of Chemical Technology, ingeniously designed a novel drug delivery system with cascading targeting functions (FAM DDS). This DDS effectively targets macrophages and intracellular methicillin-resistant Staphylococcus aureus (MRSA), enabling precise in-situ delivery of the antibiotic rifampicin (Rif), overcoming the bottleneck issues of dormant intracellular bacteria and concealed locations that weaken antibiotic efficacy. Additionally, Rif@FAM can promote M1/M2 polarization of macrophages, enhance macrophage immune clearance function, and collaboratively achieve efficient clearance of intracellular MRSA with Rif.
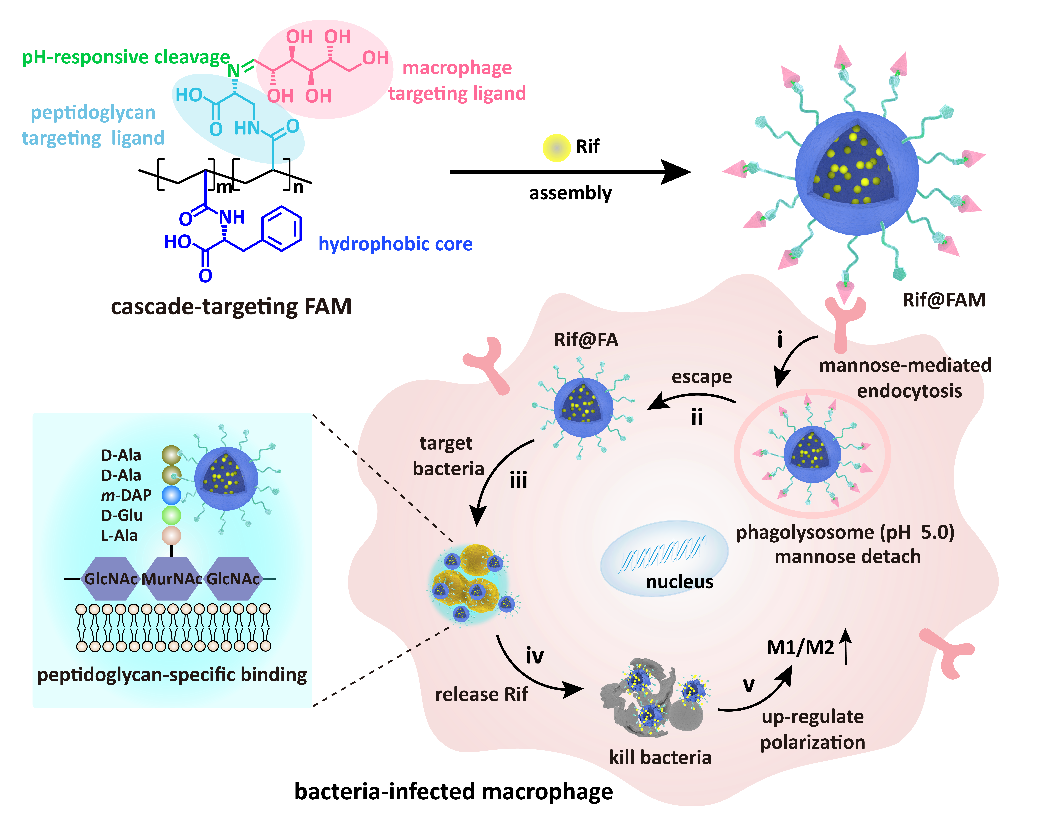
Figure 1: Rif@FAM cascade targeting clearance of MRSA within macrophages
Article Highlights:
(1) Design and synthesis of Rif@FAM DDS. The research group for the first time used β-N-acryloyl-D-amino acid as the main monomer, synthesized the polymer FA targeting bacteria specifically through photo-induced polymerization technology, utilized dynamic Schiff base to graft mannose obtaining the polymer FAM with targeting ability towards macrophages, and then self-assembled to encapsulate Rif, constructing Rif@FAM DDS with cascade targeting intracellular bacteria function (Figure 2). This strategy provides high-density intracellular bacteria targeting groups for DDS, reducing off-target effects; moreover, the rich non-covalent interactions of amino acid polymers enhance the drug loading efficiency (18.9 wt%) and acid stability of DDS, achieving sustained drug release performance for up to one month.
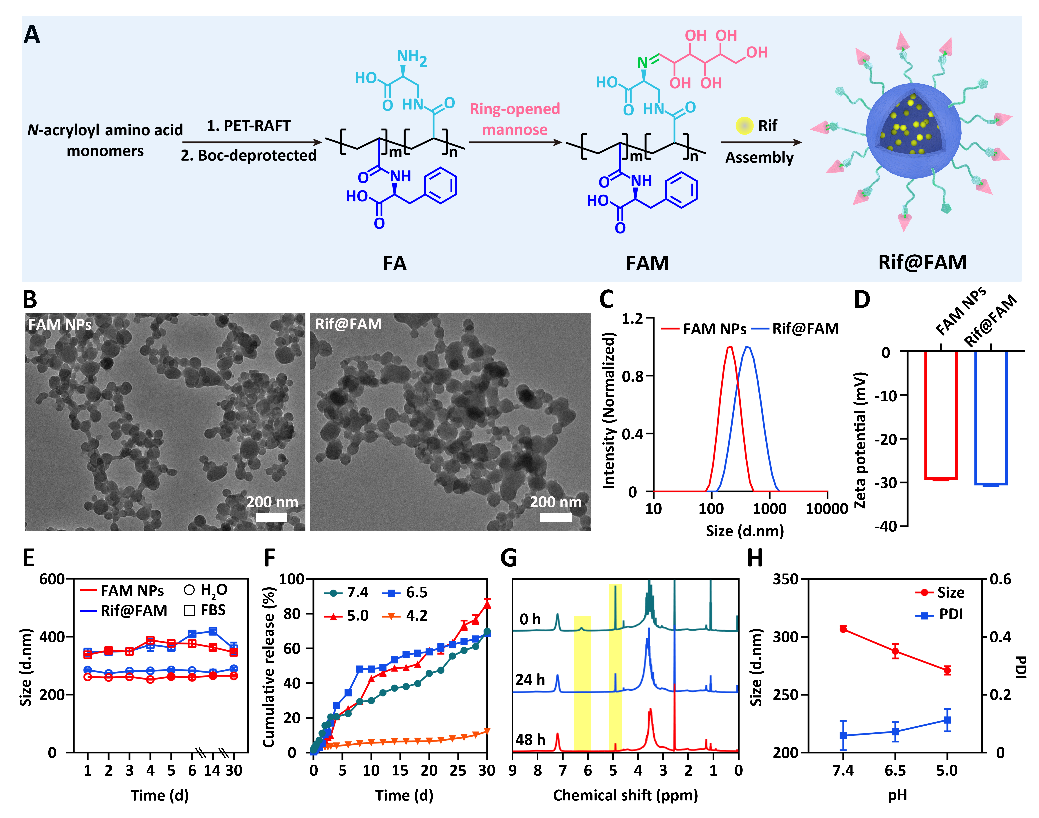
Figure 2: Synthesis and Characterization of Rif@FAM DDS
(2) Cascade Targeting Mechanisms. Research has shown that Rif@FAM first enters cells via mannose receptor-mediated endocytosis, selectively accumulating in macrophages, increasing the intracellular enrichment of Rif; subsequently, within the acidic environment of the lysosome, the Schiff base is cleaved, triggering mannose detachment to form Rif@FA; particles exposed with D-amino acids on the surface of Rif@FA escape from the lysosome and specifically bind to the bacterial wall of intracellular MRSA, thereby achieving cascade targeting. Through in situ/non-in situ co-localization techniques, the authors demonstrated the specific targeting effect of FAM DDS on intracellular MRSA (Figure 3). Three-dimensional confocal imaging shows a high overlap of fluorescence between FAM DDS and intracellular MRSA. Quantitative analysis using flow cytometry revealed a high overlap of 71.6% between intracellular MRSA collected after cell lysis and FAM, approximately 3 times higher than the control group, indicating that the high-density D-amino acids enhanced the binding capacity of FAM DDS with bacteria, effectively avoiding off-target effects. In situ TEM imaging further confirmed the binding of FAM DDS to the bacterial wall of intracellular MRSA, presenting a distinct contrast compared to the smooth bacterial wall of the control group.
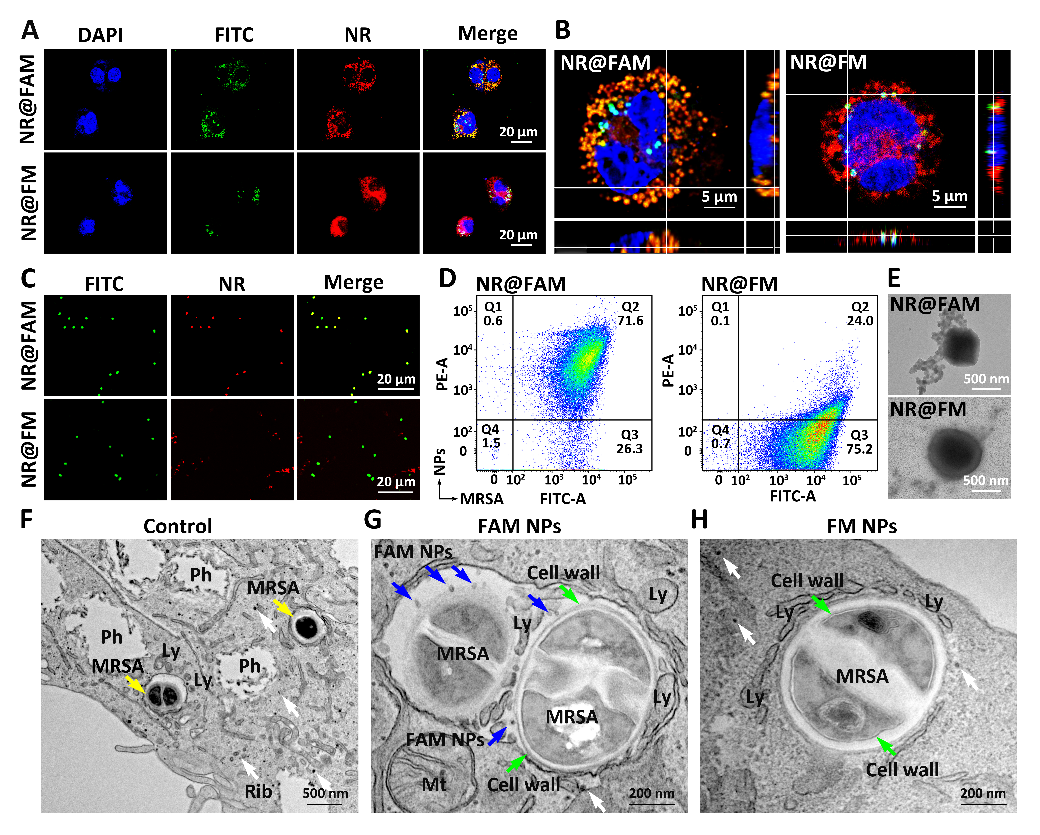
Figure 3: Targeting intracellular bacteria with FAM DDS
(3)In vivo antibacterial activity. Following the intravenous injection of FAM DDS, the tested mice demonstrated that FAM DDS could accumulate at the MRSA infection site within 24 hours, further proving the in vivo drug delivery capability of FAM DDS at the animal level (Figure 4). Furthermore, the authors compared the therapeutic effects of Rif@FAM with single-functional cell-targeting DDS (Rif@FM) and bacteria-targeting DDS (Rif@FA) on acute peritonitis in mice. The statistical data showed that the intracellular MRSA quantity in the Rif@FAM experimental group (1.36 log10CFU) was significantly lower than that in the Rif@FA control group (2.18 log10CFU; **, p = 0.004) and Rif@FM group (3.24 log10CFU; ***, p = 0.0004). These results indicate that, compared to single cell-targeting and bacteria-targeting DDS, the cascade targeting and in situ drug delivery properties of FAM DDS can significantly enhance the therapeutic effect of antibiotics.
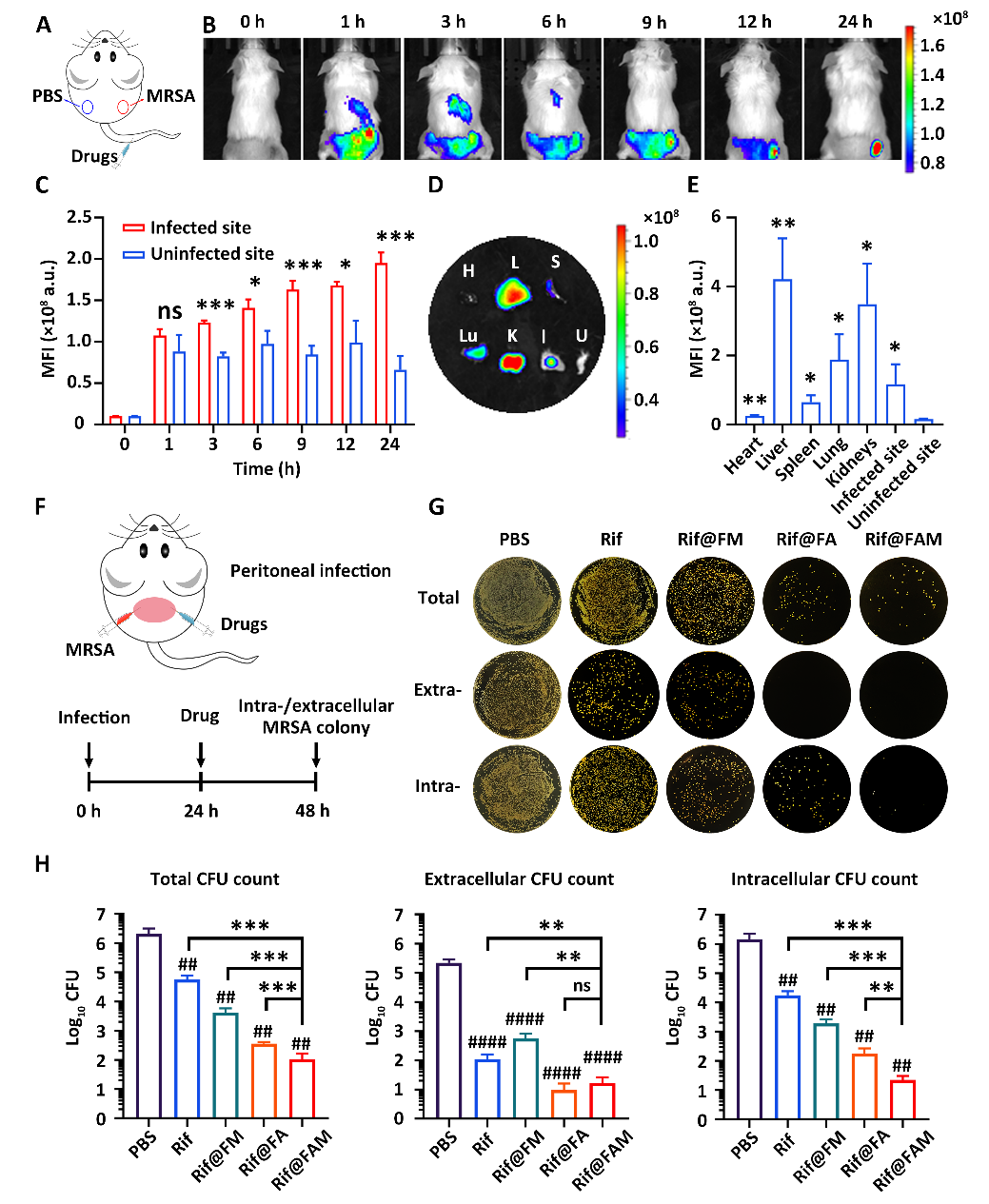
Figure 4: In vivo targeting and antibacterial performance evaluation of FAM DDS
In summary, Rif@FAM achieved efficient clearance of intracellular bacteria through cascade targeting and on-site drug delivery. Its main advantages are: (i) achieved high intracellular accumulation and prolonged intracellular retention of Rif; (ii) structurally stable under strong acid, exhibiting long-lasting antibacterial activity; (iii) precise on-site release of Rif, overcoming the bottleneck problem of antibiotic performance weakened by intracellular bacterial dormancy and hiding; (iv) reshaped the immune response of macrophages, enhancing antibacterial performance. The study demonstrated the role and significance of cascade targeting and on-site delivery strategies in eradicating intracellular bacteria, providing new methods and technologies for the treatment of relevant diseases.
The related research results were recently published under the title "Cascade-targeting Poly(amino acid) Nanoparticles Eliminate Intracellular Bacteria via on-site Antibiotic Delivery" in the journal Advanced Materials (IF = 30.849). The first author of this paper is Feng Wenli, a doctoral student at the School of Life Science and Technology, Beijing University of Chemical Technology. Professor Wang Xing and Associate Professor Li Guofeng from Beijing University of Chemical Technology are the corresponding authors of the paper. This research was supported by the National Natural Science Foundation of China, the National Major Innovative Drug Development Project, the Fundamental Research Funds for the Central Universities, and the Beijing Natural Science Foundation.
Article link: https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202109789
Keywords:
Poly(amino acid); cascade-targeting drug delivery system; intracellular bacteria targeting; on-site drug delivery; macrophage polarization
