On April 11, 2022, the research group led by Feng Yue at our School of Life Sciences published a research paper titled "Insights into the inhibition of type I-F CRISPR-Cas system by a multifunctional anti-CRISPR protein AcrIF24" online in "Nature Communications." The paper reported the three-dimensional structures of AcrIF24, AcrIF24-Csy, and two types of AcrIF24-Csy-dsDNA complexes, revealing the dual functions of AcrIF24 as an Anti-CRISPR associated protein and its multiple inhibition mechanisms on the I-F type CRISPR-Cas system.

In this study, the Feng Yue research group first elucidated the crystal structure of the central domain truncation protein AcrIF24ΔMD of AcrIF24, and through bioinformatics analysis and cellular experiments, confirmed that the C-terminal domain of AcrIF24 has the function of an Aca protein, namely, inhibiting the transcription of acr operon. Subsequently, they demonstrated that AcrIF24 can directly bind to Csy and in collaboration with the research group of Professor Yang Maojun from Tsinghua University, used cryo-electron microscopy to resolve the structure of the AcrIF24-Csy complex, revealing that an AcrIF24 dimer can bind to two Csy molecules, forming a heterotetrameric complex (Figure a). Furthermore, they found that although the binding of crRNA to target DNA in the Csy complex is hindered by the spatial presence of AcrIF24, the AcrIF24-Csy complex can still adsorb sequence non-specific dsDNA and form higher-order complexes, thereby "misleading" the Csy complex to bind to "ineffective" sites in exogenous dsDNA. They further elucidated the three-dimensional structures of two types of AcrIF24-Csy-dsDNA complexes binding to target and non-target dsDNA, and through a series of biochemical experiments, clarified the mechanism and characteristics of how AcrIF24 induces the non-specific adsorption of dsDNA by the Csy complex (Figure b).
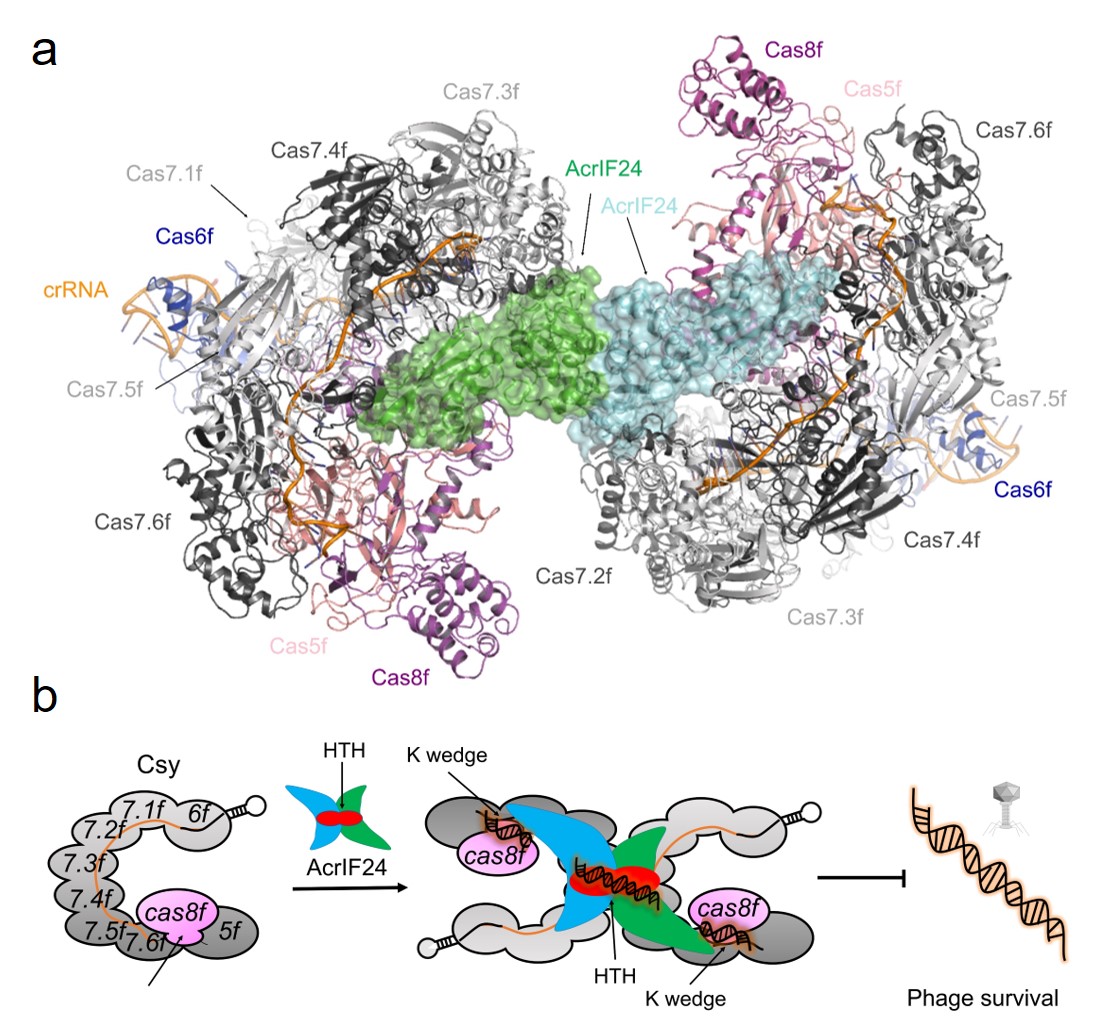
In conclusion, AcrIF24 is the first dual-function Acr-Aca protein to be structurally resolved and functionally characterized, exhibiting (1) spatial hindrance of Csy recognition target DNA; (2) formation of heterotypic tetrameric complexes with Csy; (3) and exhibiting non-specific DNA adsorption. Elucidation of its mechanism provides insights for the study of other Acr-Aca proteins and establishes a foundation for developing it as a regulatory tool for I-F type CRISPR-Cas gene editing. Notably, the research group led by Yue Feng reported AcrIF14 in 2021, as well as AcrIF9 reported by Blake Wiedenheft and Alan R Davidson's research group, both showing stable binding to Csy to prevent target dsDNA recognition while inducing nonspecific ineffective DNA adsorption. This suggests that inducing nonspecific ineffective DNA adsorption may be common among various Acr proteins.
Dr. Lingguang Yang (postdoc, Beijing University of Chemical Technology, departed), Dr. Laixing Zhang (postdoc, Tsinghua University), Dr. Peipei Yin (postdoc, Beijing University of Chemical Technology, departed), and master student Hao Ding are the co-first authors of this paper, with Professor Yue Feng as the corresponding author. Professor Maojun Yang from Tsinghua University provided significant guidance in electron microscopy structure analysis, with electron microscopy data and crystal diffraction data collection supported by the Cryo-Electron Microscopy Center of Southern University of Science and Technology and the Shanghai Synchrotron Radiation Facility.
Original article link: [Article Link](https://www.nature.com/articles/s41467-022-29581-1)
