Based on the cyclic-oligonucleotide-based antiphage signaling system (CBASS), which is an important immune system of bacteria against phage infections, bacteriophages encounter resistance from the bacterial immune system post-infection. Bacteriophages employ various strategies to counteract the bacterial immune system, with the most well-understood being encoding multiple proteins to inhibit host immune systems like CRISPR-Cas and restriction modification systems. However, it remains unclear whether bacteriophages have mechanisms to inhibit or evade the CBASS immune system.
On February 6, 2023, our research group led by Feng Yue from the school collaborated with Joseph Bondy-Denomy's research group from the University of California, San Francisco, and published a research paper titled "Bacteriophages inhibit and evade cGAS-like immune function in bacteria" online in Cell, detailing the mechanisms through which bacteriophages inhibit and evade the CBASS immune system in bacteria.
Through bioinformatics analysis, a total of 252 different strains containing the CBASS system in P. aeruginosa were screened. The CBASS gene locus was knocked out using CRISPR-Cas technology, resulting in the selection of a strain, P. aeruginosa BWHPSA011 (Pa011), with a naturally functional CBASS immune system. This strain contains a cGAS-like enzyme, CdnA, which produces 3’, 3’-cGAMP in response to PaMx41 phage infection, activating the phospholipase (CapV) effector protein to exert the immunological function of the CBASS system. The escape mutants of PaMx41 phage can produce a protein, Acb2, that is resistant to the CBASS system. How does Acb2 exert its resistance function? Professor Feng Yue's research team combined various methods such as biochemistry and structural biology to elucidate the functional mechanism of Acb2 in inhibiting the CBASS system. They found that Acb2 can efficiently bind to 3’, 3’-cGAMP molecules but does not have enzymatic activity. The research team resolved the structure of the Acb2 protein and its complex with 3’, 3’-cGAMP, revealing that the Acb2 protein itself forms a compact hexamer structure that can bind to three 3’, 3’-cGAMP molecules. Each 3’, 3’-cGAMP molecule is located in a "binding pocket" formed by two Acb2 monomers at the N-terminus, leading to stable interactions. By sequestering and isolating 3’, 3’-cGAMP, Acb2 effectively disrupts the immunological function of the CBASS system. Subsequent in vitro biochemical experiments further confirmed that Acb2 can also bind to various oligonucleotide signaling molecules, including c-di-AMP/3’, 3’-c-di-UMP/2’, 3’-cGAMP/3’, 3’-cUA/UG, covering both Type I and Type II CBASS systems. This indicates that Acb2 is a broad-spectrum inhibitory protein of the cGAS enzyme-based immune system. Additionally, it was discovered that the absence of Acb2 leads to the inhibition of phage replication and lysogen induction by the CBASS system, although a few phages can evade the immune response of the CBASS system through mutations in major capsid genes (Fig.1).
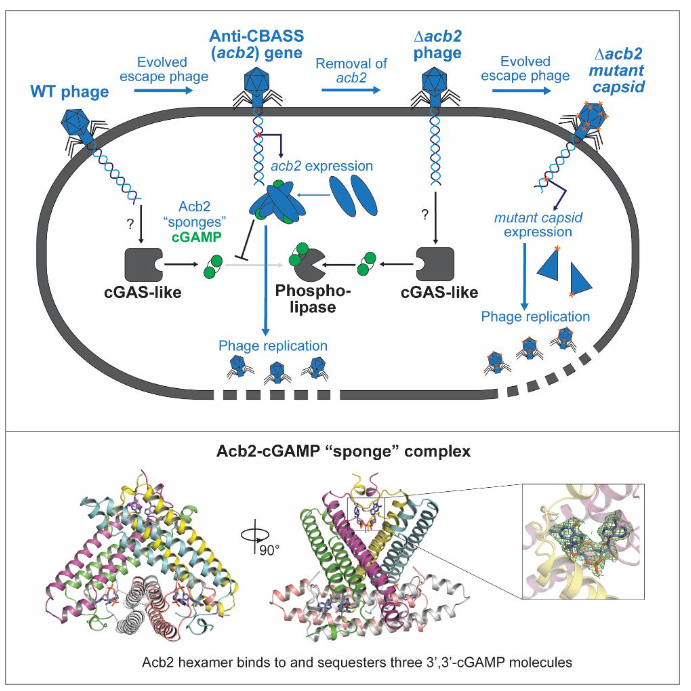
Fig.1 Mechanism of action of the anti-CBASS system of Acb2 protein
The article reveals the antibacterial phage immune function of the endogenous CBASS system and proposes effective strategies to evade and inhibit phage immune system recognition. The broad-spectrum inhibitory protein Acb2 can inhibit various types of CBASS systems by binding to various cyclic oligonucleotides. The first authors of this paper are Erin Huiting, a doctoral student at the University of California, San Francisco, and Xueli Cao, a doctoral student of our university. Professor Yue Feng from our university and Professor Joseph Bondy-Denomy from the University of California, San Francisco, are the corresponding authors of this paper.
Original link: https://www.sciencedirect.com/science/article/pii/S0092867422015847
Author's bio: Yue Feng, professor of the School of Life Science, Beijing University of Chemical Technology, doctoral supervisor, recipient of the National Excellent Youth Fund (2018). He mainly conducts research on the structure and function of proteins related to microbial and host immune system interactions using biochemistry, molecular biology, structural biology, and cell biology. He has published 44 SCI papers, including 22 corresponding author papers, in internationally renowned journals such as Nature, Cell, Mol. Cell, Nat. Chem. Biol., PNAS, Nat. Plants, Nat. Commun., Nucleic Acids Res., J. Biol. Chem., etc. He has hosted multiple national and provincial-level projects and has received honors such as National Young Skilled Talent (2020), Outstanding Young Talent in Beijing (2020), Second Prize of Beijing Science and Technology Progress Award (2018), Top Ten Emerging Science and Technology Figures in China (2018), Beijing Science and Technology Star (2019), etc.
