The chloroplast originated from endosymbiosis within cyanobacterial cells, serving as a unique photosynthetic organelle in plants. Enclosed by a double membrane, it continues to divide like an independent entity[1]. During chloroplast division, a protein complex forms at the division site, with FtsZ related to microtubule proteins acting as the scaffold protein of the division site complex. The assembly of FtsZ proteins forms oligomers, creating a ring structure at the division site. The formation of the FtsZ ring is regulated by the Min system to ensure only one FtsZ ring forms at the center of the chloroplast. Within the chloroplast, FtsZ is anchored to the inner membrane through the C-terminal domain of FtsZ2 and the N-terminal domain of ARC6, while the C-terminal domain of ARC6 interacts with the C-terminal domain of the outer membrane protein PDV2. The plant Min system also includes various regulatory proteins such as MCD1, ARC3, and PARC6. PARC6, also known as CDP1, as a homologous protein of ARC6, exhibits distinct functions during chloroplast division. The N-terminal domain of PARC6 is located on the matrix side of the chloroplast, interacts with ARC3, and enhances its function. ARC6 recruits PDV2 to the chloroplast division site, where PDV2 also influences the assembly of chloroplast division protein complexes. In March 2017, Professor Feng Yue's research group at our university collaborated with Professor Gao Hongbo's research group at Beijing Forestry University to publish a paper in "Nature Plants" that reported on the structure of ARC6 and PDV2, as well as their molecular mechanisms in regulating chloroplast division[2].
After a gap of six years, in January 2023, Professor Feng Yue's research group at our university once again collaborated with Professor Gao Hongbo's research group at Beijing Forestry University to publish a research paper titled "Structural and functional insights into the chloroplast division site regulators PARC6 and PDV1 in the intermembrane space" in PNAS. The study revealed a multi-layer regulatory mechanism of the PDV1-PARC6 interaction, enhancing our understanding of the chloroplast division process.
Previous studies suggested that PDV1 and PDV2 collaborate to recruit ARC5 to the chloroplast division site. Overexpression of PDV1 does not inhibit chloroplast division, but slightly promotes it. The interaction between PDV1 and ARC5 supports the idea that PDV1 assists in recruiting ARC5 to the division site. However, subsequent findings showed that GFP-ARC5 can localize to PDV1 mutants with severe chloroplast division defects, indicating that PDV1 may have other roles besides recruiting ARC5 to the chloroplast division site. Professor Gao Hongbo's research team verified the interaction between PDV1 and PARC6 proteins through in vivo experiments. Professor Feng Yue's research team combined various methods such as biochemistry and structural biology to reveal the multi-layer regulatory roles of the PARC6-PDV1 complex in the intermembrane space and chloroplast division. Firstly, Professor Feng Yue's team elucidated the structure of the PARC6 protein and its complex with PDV1. The oligomeric state of PARC6 is regulated under redox conditions: under oxidative conditions, PARC6 exists as a dimer due to the presence of intramolecular disulfide bonds, and a helical lid suppresses the interaction between PARC6 and PDV1; under reducing conditions, the opening of disulfide bonds leads to the opening of the binding pocket of PARC6, allowing PDV1 to insert into PARC6's binding pocket. Interestingly, in the reducing state, when PARC6 interacts with PDV1, PDV1 promotes the formation of PARC6 dimers. The stability of the PDV1-PARC6 complex is not only regulated by redox conditions but also influenced by magnesium ion concentration, both of which are light-regulated. Professor Gao Hongbo's research team validated these conclusions through in vivo experiments.
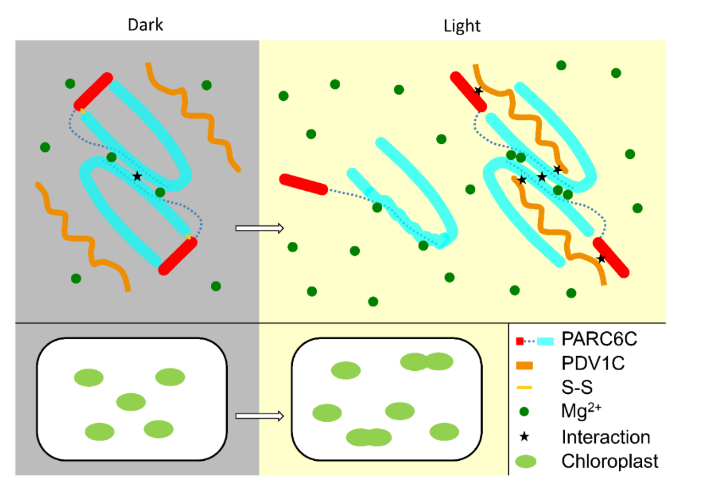
Fig.1 Regulatory mechanism of PARC6–PDV1 complex
In conclusion, this study reveals the multi-layer regulation of the PARC6-PDV1 complex in the intermembrane space and chloroplast division, expanding our understanding of the Min system regulation mechanism in the chloroplast division apparatus. This paper is co-first authored by Cao Xueli, a doctoral student at Beijing University of Chemical Technology, Liu Zihe, a graduate master's student, as well as Dr. Sun Qingqing and Dr. An Chuanjing from Beijing Forestry University. This work is co-corresponding authored by Professor Feng Yue from Beijing University of Chemical Technology and Professor Gao Hongbo from Beijing Forestry University.
1.K. W. Osteryoung, J. Nunnari, The division of endosymbiotic organelles. Science 302, 1698-1704 (2003).
2.W. H. Wang et al., Structural insights into the coordination of plastid division by the ARC6-PDV2 complex. Nat Plants 3, (2017).
